pH & Pharmacokinetics
Effect of Altering pH
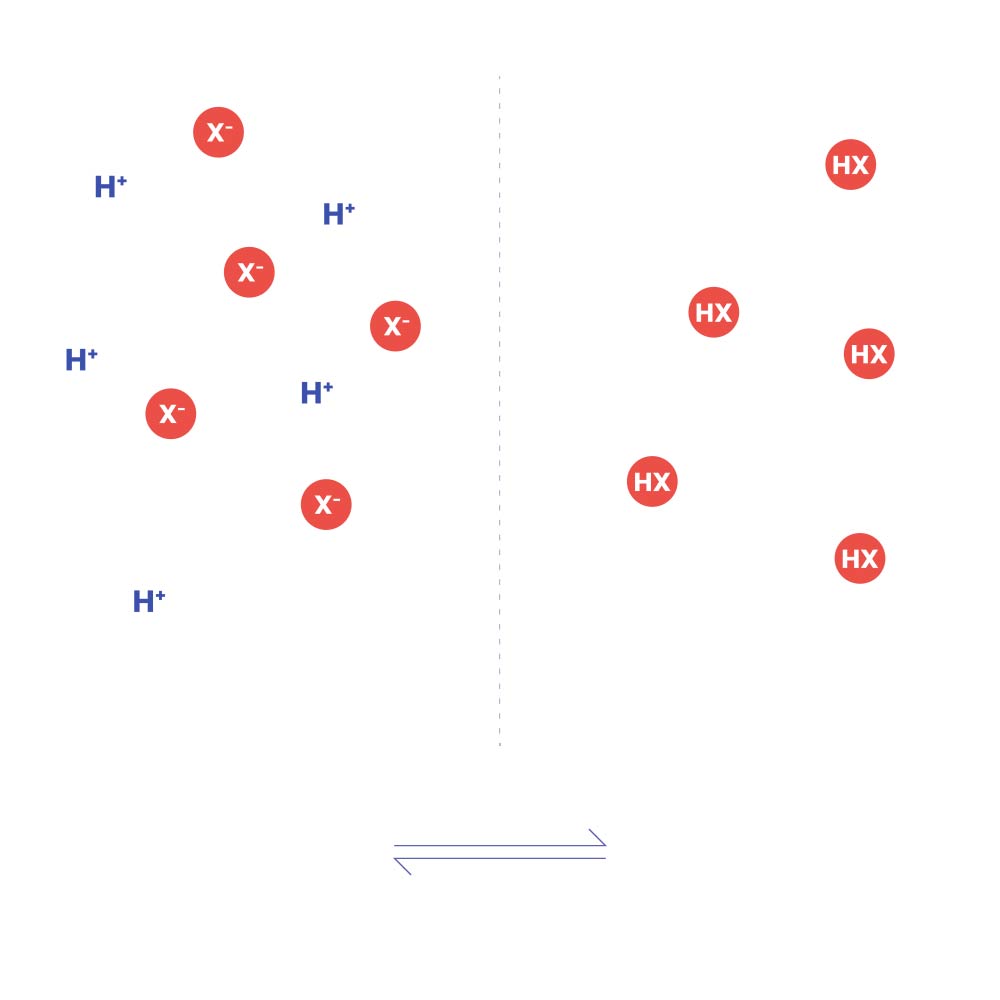
If acid is added, eg. H⁺Cl⁻, the equilibrium moves to the right. If alkali is added, eg. Na⁺OH⁻, then OH⁻ ions and H⁺ ions neutralise each other to form water, and the equilibrium moves to the left.
Unionized drug crosses lipid biological barriers (e.g. membranes) better than ionized drug.
Add acid or alkali and observe what happens to the equilibrium.
 Reset
Reset
H⁺+X⁻
HX
membrane
(ionized)
(unionized)
ACIDS ARE IONIZED IN BASIC MEDIA
BASES ARE IONIZED IN ACIDIC MEDIA